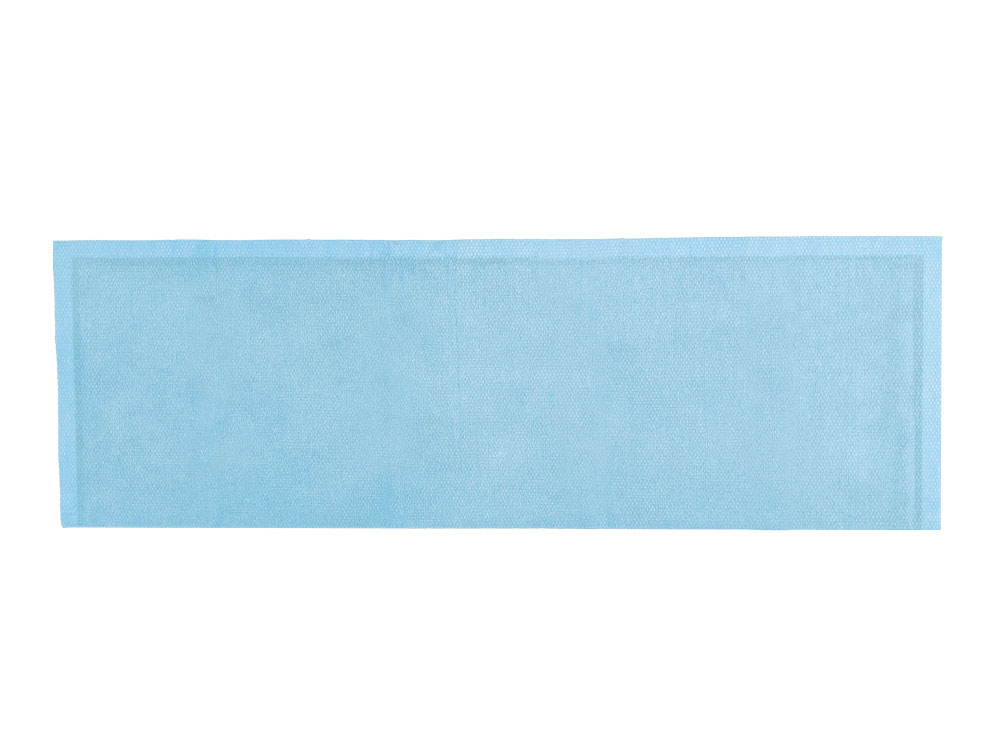
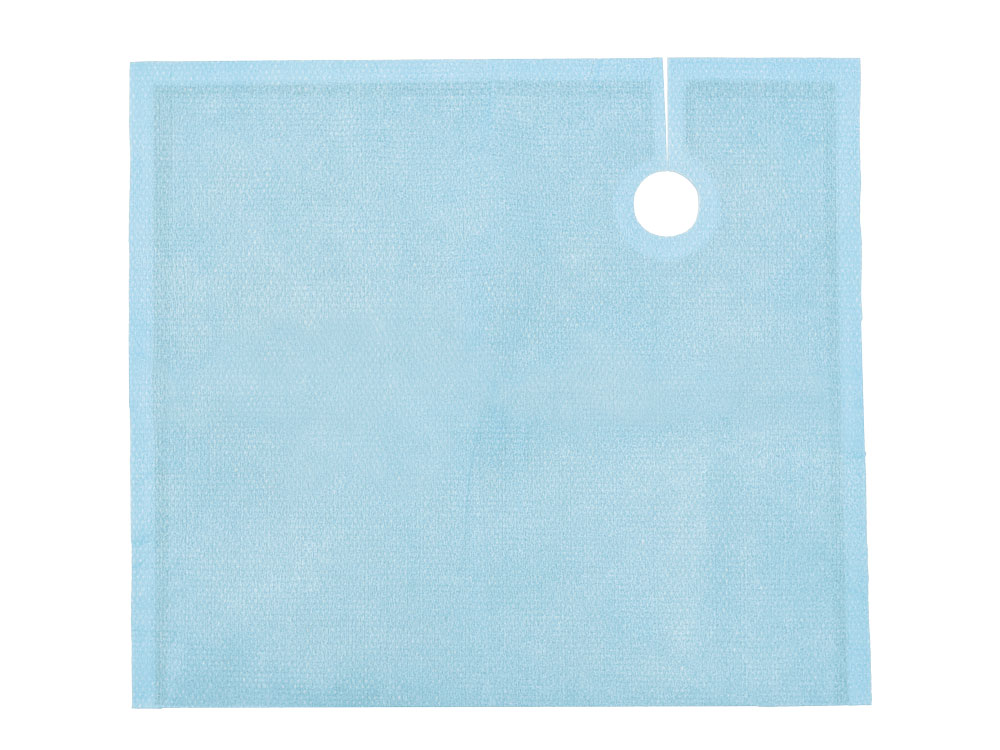
Peripheral Drape
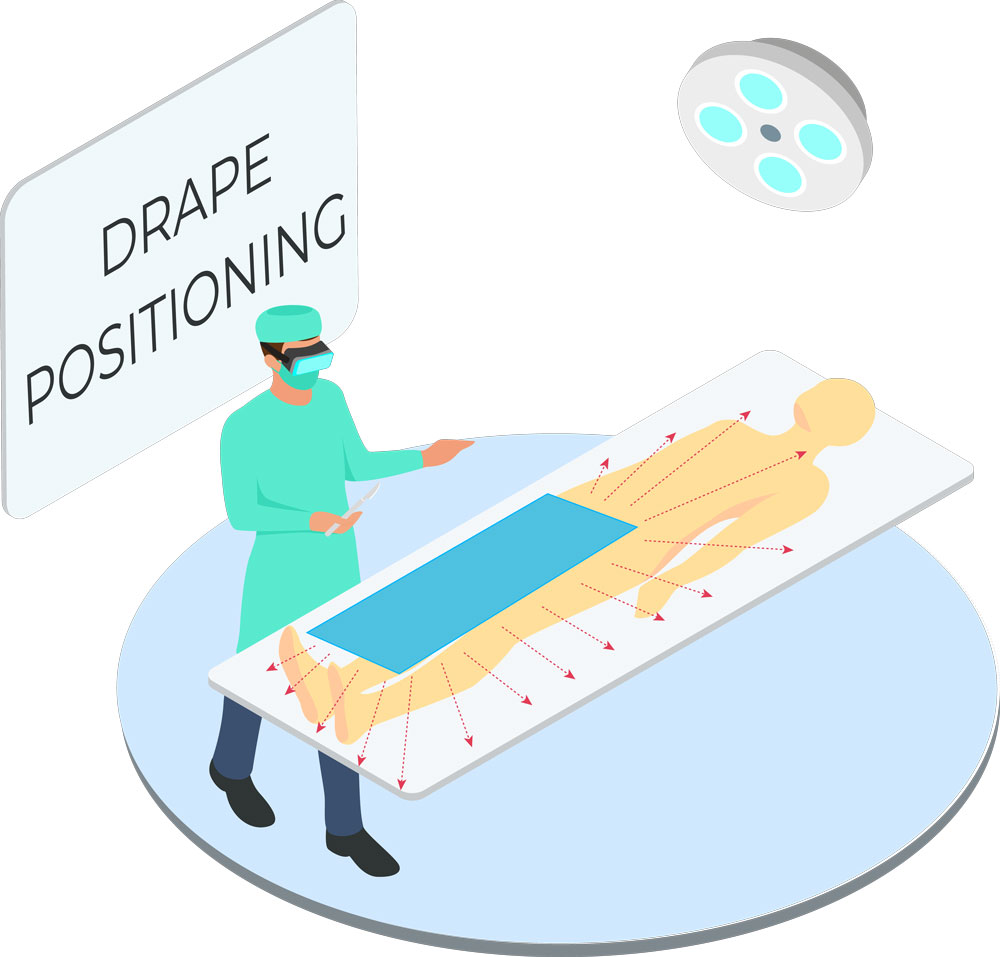
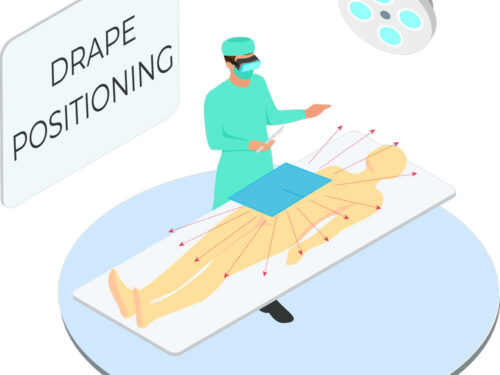
Tested in accordance with ASTM standards, ScatterGuard Peripheral patient drapes with Fenestration provide excellent protection for fluoro-lab and operating suite personnel during interventional procedures. These drapes are placed over the patient, so you benefit from added protection without the added weight! They are highly absorbent, sterile, disposable through normal waste procedures, conform to the patient’s body, and feature 3M adhesive backing to prevent slippage.
Minimum Order: 10 drapes (1 box)
Product Information
Sterile
Disposable
Eco Friendly
Latex Free
3M Adhesive
High Absorbency
- Dimensions: 11″ x 34″
- Fenestration: None
- Core Material: Prolite Max
- Protection Options: 0.125mm, 0.25mm, 0.375mm LE
- Shelf Life: Sterile – 3 years after manufacture date
- Quantity: 10 drapes per box
- Fabric: ActiV – highly absorbent
- CE Marked: Yes (only applies to 0.25mm LE)
- Certifications: Certified to ASTM F2547-18 standards.
IEC 61331-1:2014 certified drapes available upon request. - For Use During: Peripheral procedures where access is through the femoral artery
| Lead Equivalence | Attenuation @ 90kV |
|---|---|
| 0.125mm | 75% |
| 0.250mm | 90% |
| 0.375mm | 95% |
| Fabric | Weight | Absorbency Rate | Absorbency Capacity |
|---|---|---|---|
| ActiV Absorbent | 120 gsm | 1.2 sec/ml | 620% |
| Leading Competitor 2 | 130 gsm | 2.5 sec/ml | 600% |
| Leading Competitor 1 | 109 gsm | 1.9 sec/ml | 526% |
Compliance Requirements: ASTM F2547-18
Biocompatibility Compliance: ISO 10993, ASTM F719 and ASTM F720
Disposal: Scatterguard Drapes should be disposed of through normal waste procedures in accordance with local laws and regulations. The material should not be disposed of by incineration and use of self-contained breathing apparatus is recommended if the sheeting is ignited by fire.
Expiration: This is a sterilized product and expiration is set at 3 years from manufactured date per FDA requirement. This indication may be found on the label on the original external packaging and indicates the period of time during which the device, sterilized with Gamma Radiation, may be used as long as it has been stored properly in suitable conditions and has intact packaging.
You may also like…
Price range: $195.00 through $385.00
Select options This product has multiple variants. The options may be chosen on the product page$315.00
Select options This product has multiple variants. The options may be chosen on the product pagePrice range: $195.00 through $385.00
Select options This product has multiple variants. The options may be chosen on the product page

 Instructions for Use
Instructions for Use Drape Brochure
Drape Brochure Test Reports
Test Reports





Reviews
There are no reviews yet.