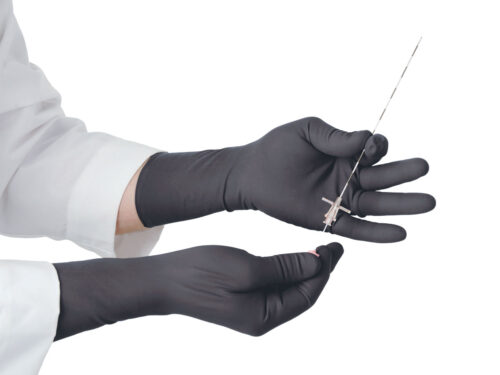
Proguard RR Gloves
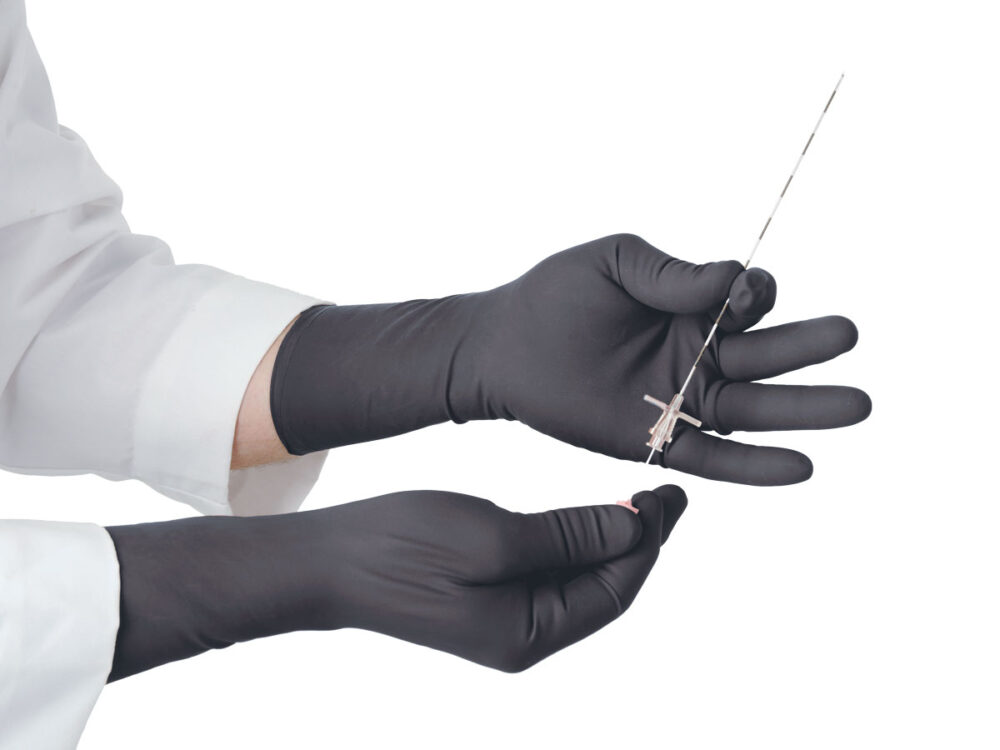
The Proguard RR radiation attenuating gloves are intended to reduce the amount of scattered radiation exposure to the hands during fluoroscopic procedures.
Certified to ASTM 2547 and IEC 61331-1:2014 Standards
Proguard radiation attenuating gloves are the preferred choice for doctors in the following vocations: Radiology, Cath/EP Lab, Orthopedics, Endoscopy, Urological Procedures, Special Procedures, and Pain Management.
Price is for 1 box (5 pairs)
Product Information
Sterile
Single Use
Premium Lead
Latex Free
Powder Free
Highly Tactile
- Material: Lead Oxide (PbO) and Dry Natural Rubber
- Allergens: Latex Free and Powder Free
- Internal Slipcoat: Polymer Coated
- Sterility: Sterilized by Ethylene Oxide
- Post Processing: Polymer Rinsing
- Shelf Life: 3 Years as per ASTM 7160-05
- Glove Design: Hand Specific, non-beaded cuff
- Glove Length: 280mm (minimum)
- CE Certified: Yes
- Packaging: Pouch (1 Pair); Box (5 Pairs); Carton (20 Pairs)
| MODEL | LEAD EQUIVALENCE | THICKNESS | AREA DENSITY | 60kVp | 90kVp | 120kVp |
|---|---|---|---|---|---|---|
| RR1 | 0.025mm Pb @ 60kVp | +/- 0.24mm | 0.45 kg/m2 | 45% | 33% | 26% |
| RR2 | 0.031mm Pb @ 60kVp | +/- 0.28mm | 0.63 kg/m2 | 52% | 40% | 32% |
| RR3 | 0.040mm Pb @ 60kVp | +/- 0.38mm | 0.63 kg/m2 | 60% | 47% | 39% |
Compliance Requirements: ASTM D5151, ASTM D6124, ASTM D412, ASTM F2547, EN ISO 374-1:2016, EN ISO 374-5:2016, EN 421:2010 (excl. clause 4.3) EN 420:2003 + A1:2009
Biocompatibility Compliance: ISO 10993, ASTM F 719 and ASTM F 720
Disposal: The Proguard RR glove contains lead and should be disposed of through hazardous waste procedures in accordance with local laws and regulations. The material should not be disposed of by incineration and use of self-contained breathing apparatus is recommended if the sheeting is ignited by fire.
Expiration: This is a sterilized product and expiration is set at 3 years from manufactured date per FDA requirement. his Indication may be found on the label on the original external packaging and indicates the period of time during which the device, sterilized with Ethylene Oxide, may be used as long as it has been stored properly in suitable conditions and has intact packaging.
You may also like…
Price range: $79.00 through $99.00
Select options This product has multiple variants. The options may be chosen on the product pageOriginal price was: $285.00.$176.00Current price is: $176.00.
Select options This product has multiple variants. The options may be chosen on the product pagePrice range: $147.00 through $240.00
Select options This product has multiple variants. The options may be chosen on the product page





 Instructions for Use
Instructions for Use MSDS
MSDS Test Reports
Test Reports








Reviews
There are no reviews yet.